In this post we overview technical considerations for clean room fan filter unit selection installation and features.
Iso 14644 clean room environments for medical devices.
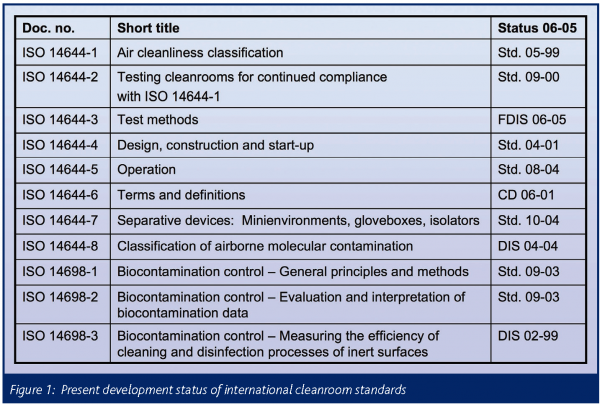
A list of all parts in the iso 14644 series published under the general title cleanrooms and associated controlled environments can be found on the iso website.
The regulatory review and approval process for medical devices in the european union eu the u s.
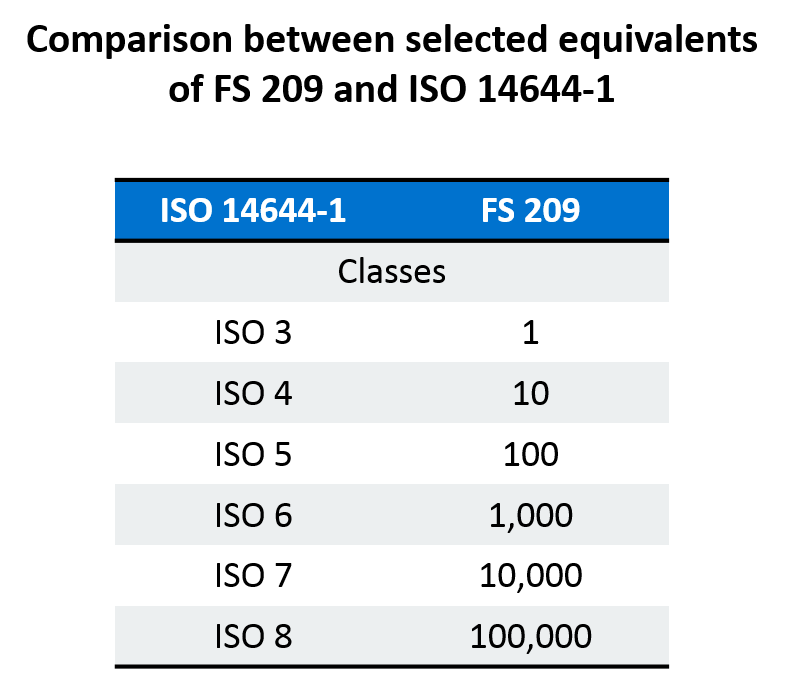
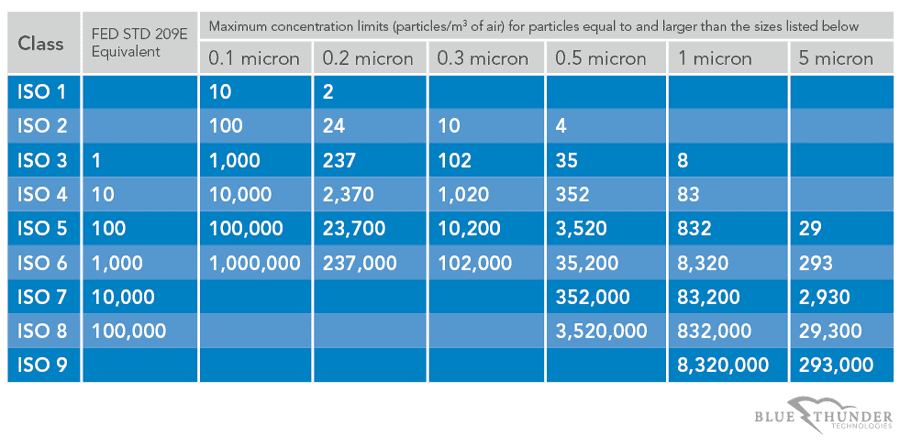
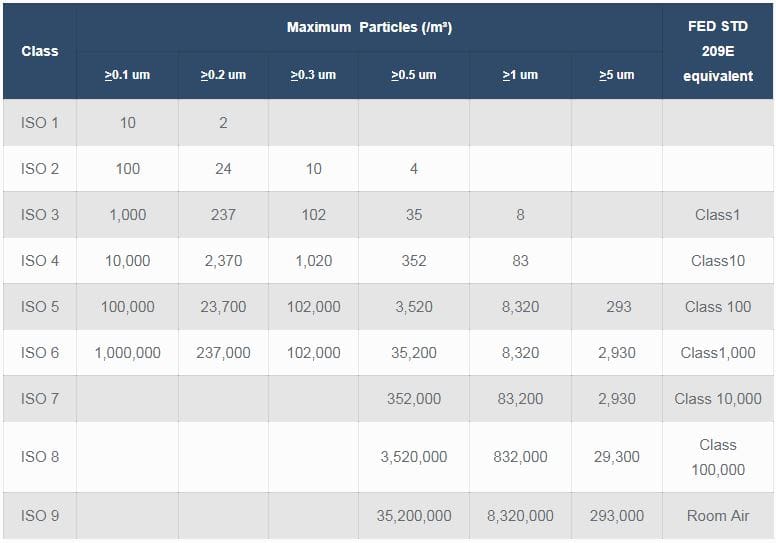
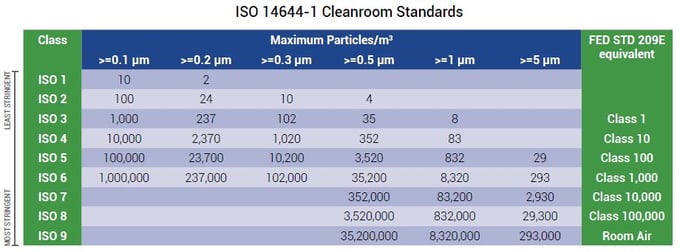
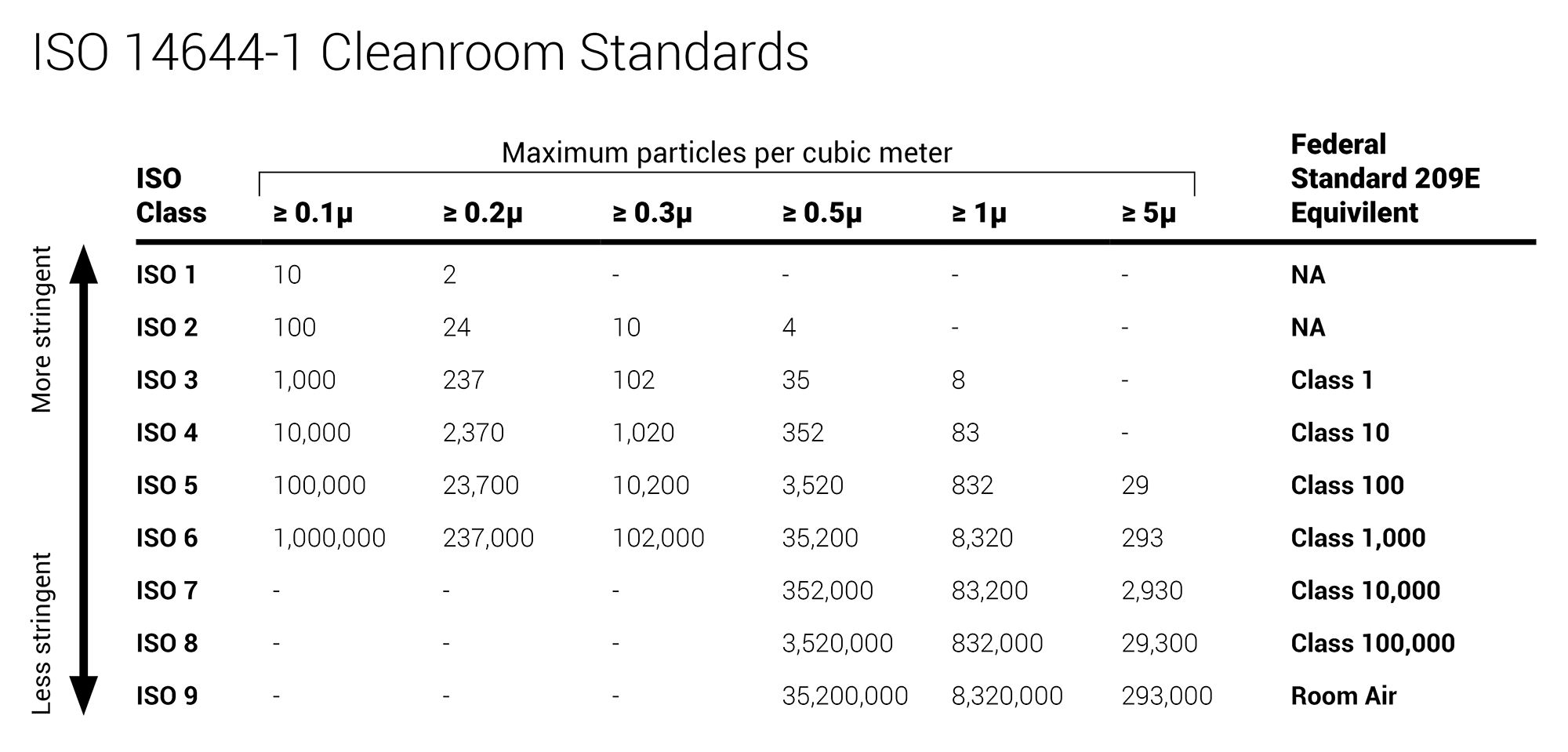
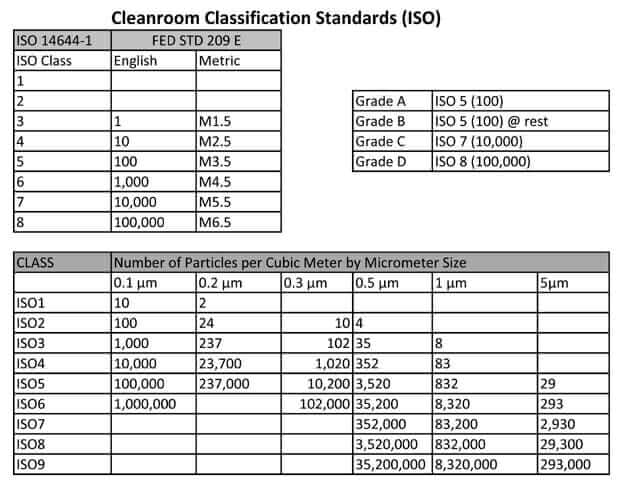
Broadly medical device manufacturing is conducted in an iso 5 8 cleanroom class 100 100 000.
Medical device cleanroom construction part 3.
What is iso 14644 cleanroom environments for medical devices.
Fan filter units ceiling grids.
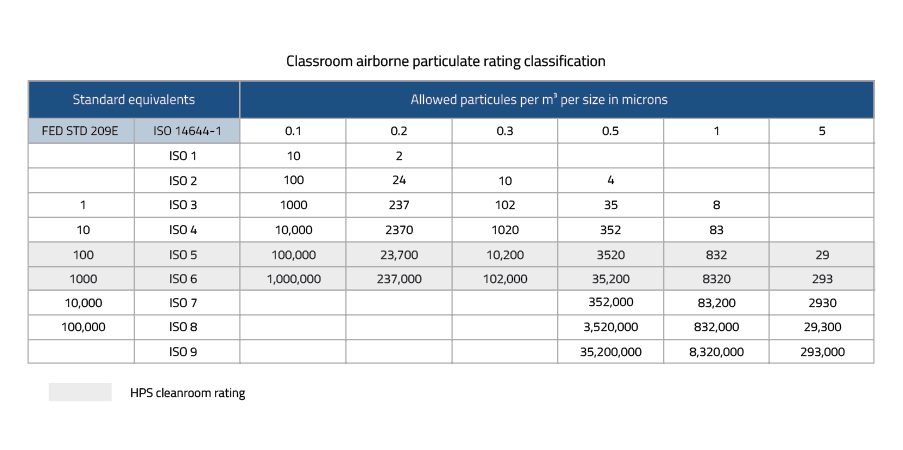
Cleanroom classification iso 14644 a cleanroom consists or either a single room or a number of interconnected rooms where the concentration of airborne and work surface particles are known and limited to pre defined levels in addition to the control of related environmental factors such as viable and non viable particles temperature air pressure airflow.
And many other jurisdictions requires manufacturers to provide evidence that their production and manufacturing facilities are designed and operated to ensure that finished products consistently meet the manufacturer s specifications.
Medical device packaging is conducted in an iso class 7 8 cleanroom.
Requirements of iso 13485 2016 for work environment and cleanliness in organizations who produce or sell medical devices ensure patients safety.
Authoritative documents such as iso 14644 and fs 209e provide no specific instructions regarding activities carried out in medical device environments.
Cleanroom environments used for the production of medical devices are expected to meet the design specifications detailed in iso 14644 4 cleanrooms and associated controlled environments part 4.
Introduction cleanrooms and associated controlled environments are widely used in many industries such as life sciences including pharmaceutical medical device and hospital.
Design construction and start up and iso 14644 5 cleanrooms and associated controlled environments part 5.
Separate lists with justifications for each category of devices make the organizational knowledge strong and unambiguous on cleaning requirements for each and every product.
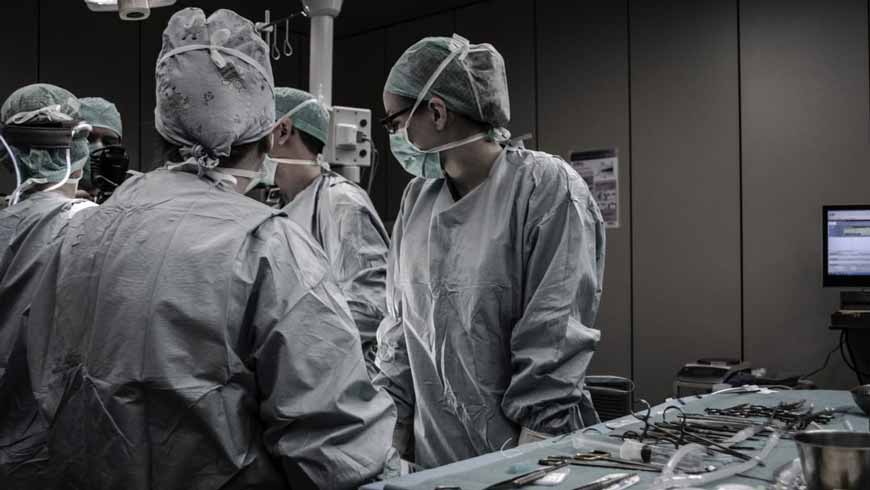
If you are into the medical industry or if your work nature is into the commercial medical or industrial industry then you should know about the clean room where every medical industry must work under clean conditions as an extremely small dust particle or off gassing can make the entire batch of medicines unusable.