In total there are a few very fda approved live stem cell products and these are only allowed for restricted use such as for bone marrow transplantation in severe end stage cancer etc.
Is amniotic stem cell therapy fda approved.
Cure blindness heal all organs associated with the stem cell therapy.
There are currently no fda approved amniotic stem cell therapy products.
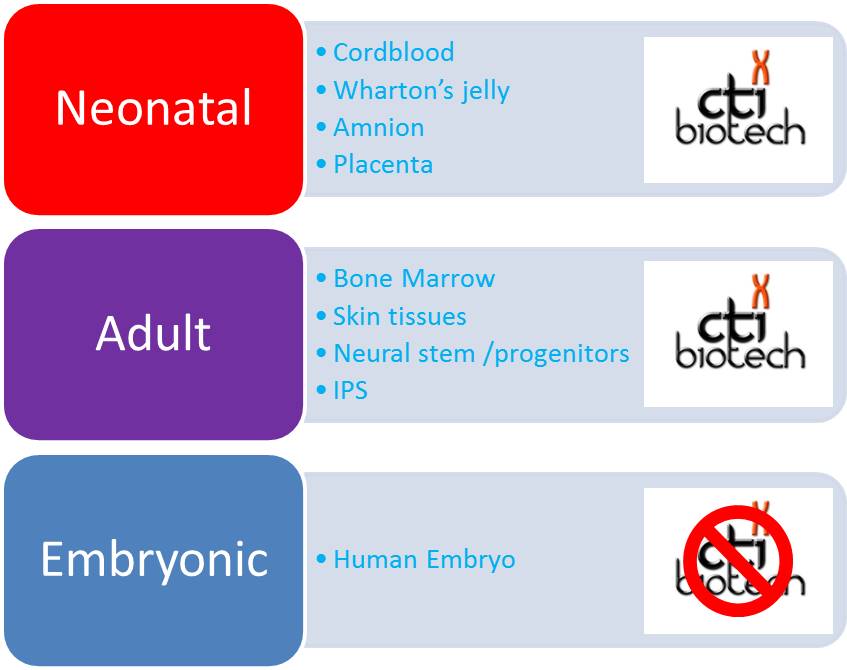
This is an installment in our continuing series on amniotic and cord stem cell products.
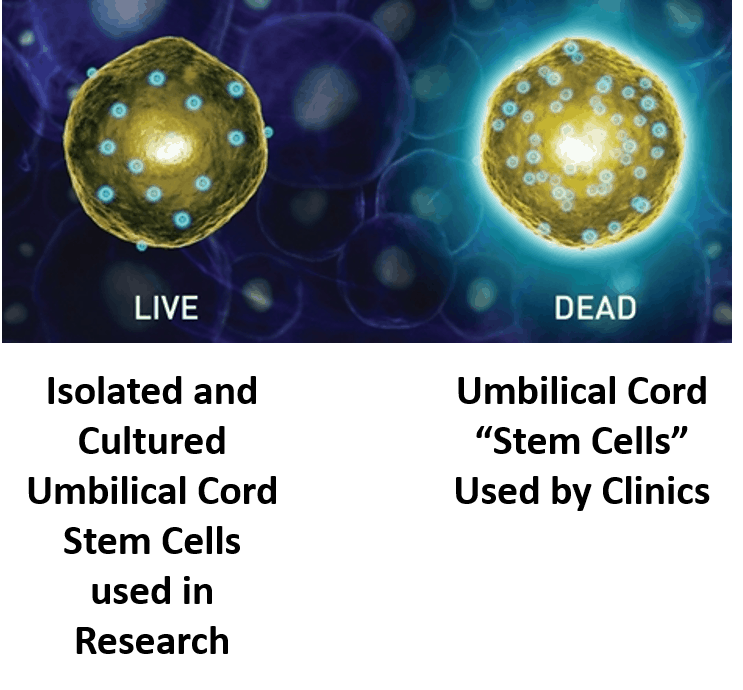
If there were any living stem cells in donated amniotic tissues to be processed for orthopedic use in another patient that would require an fda designation as a cellular drug.
In addition hopefully you now understand that anybody claiming to be selling you a stem cell treatment with living amniotic or umbilical cord stem cells is running a scam.
Now you know that amniotic or umbilical cord cells are not fda approved.
The clinics treated patients with amniotic stem cells derived from the amniotic fluid of women who had cesarean sections.
This means that the stem cell products would have to go through lengthy and very expensive clinical trials in order to potentially qualify for approval.
Some clinics also may falsely advertise that fda review and approval of the stem cell therapy is unnecessary.
But when clinical trials are not conducted under an ind it means that the fda has not.
The full list of fda approved products is here.
In fact fda has a warning on their website about this issue.